Ap Biology Lab 01 Diffusion and Osmosis Video Review Sheet Answers
The cell membrane plays the dual roles of protecting the living cell past acting as a barrier to the exterior world, yet at the same time information technology must permit the passage of nutrient and waste product products into and out of the jail cell for metabolism to go on. How does the cell conduct out these seemingly paradoxical roles? To understand this process you need to sympathize the makeup of the prison cell membrane and an important phenomenon known as diffusion.
Diffusion is the movement of a substance from an area of loftier concentration to an area of depression concentration due to random molecular movement. All atoms and molecules possess kinetic free energy, which is the free energy of movement. It is this kinetic free energy that makes each atom or molecule vibrate and motion around. (In fact, y'all can quantify the kinetic energy of the atoms/molecules in a substance by measuring its temperature.) The moving atoms bounce off each other, like bumper cars in a carnival ride. The movement of particles due to this energy is chosen Brownian motility. As these atoms/molecules bounce off each other, the result is the move of these particles from an area of loftier concentration to an area of low concentration. This is improvidence. The rate of diffusion is influenced by both temperature (how fast the particles move) and size (how big they are).

Role 1: Brownian Movement
In this part of the lab, you volition apply a microscope to observe Brownian motion in carmine red powder, which is a dye obtained from the pulverized guts of female cochineal beetles.
Materials
- Glass slide
- Toothpick
- Carmine reddish pulverization
- Coverslip
- Tap h2o
Process
- Obtain a microscope slide and place a drib of tap water on information technology.
- Using a toothpick, advisedly add together a very minuscule quantity of ruby red powder to the drop of water and add a coverslip.
- Observe under scanning, low, and and so high power.
Lab Questions
- Depict the activity of the cherry scarlet particles in water.
- If the slide were warmed upwardly, would the rate of motility of the molecules speed up, dull down, or remain the same? Why?
Role 2: Diffusion across a Semipermeable Membrane
Considering of its structure, the cell membrane is a semipermeable membrane. This ways that SOME substances tin easily diffuse through it, like oxygen, or carbon dioxide. Other substances, similar glucose or sodium ions, are unable to pass through the cell membrane unless they are specifically transported via proteins embedded in the membrane itself. Whether or not a substance is able to lengthened through a jail cell membrane depends on the characteristics of the substance and characteristics of the membrane. In this lab, we volition brand dialysis tubing "cells" and explore the outcome of size on a molecule'southward ability to diffuse through a "cell membrane."
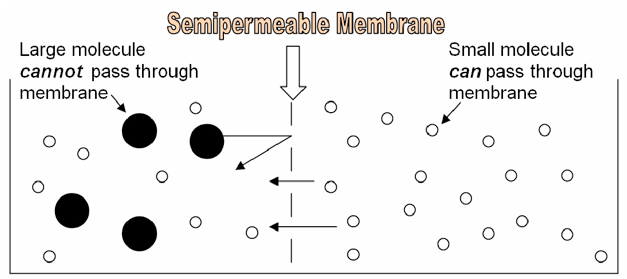
The following data might be useful in understanding and interpreting your results in this lab:
- Phenolphthalein
- Atomic formula: C20HxivO4
- Atomic mass: 318.32 thousand/mol
- Color in acidic solution : Clear
- Color in basic solution: Pink
- Iodine
- Atomic formula: I or I2
- Materials
- Atomic mass: 126 thousand/mol
- Starch
- Atomic formula: (Chalf dozenH10Ofive)northward
- Atomic mass: HUGE!
- Color in Iodine: Bluish
- Sodium Hydroxide
- Diminutive formula: NaOH
- Diminutive mass: 40.1 g/mol
- Acrid/Base of operations: Base
Materials
- 2 pieces of dialysis tubing
- Thread
- Phenolphthalein
- Iodine
- Wax pencil
- 2 beakers
- NaOH
- Starch solution
- Pipettor
- Pipette
Procedure
- Using a wax pencil, label ane beaker #1. Characterization the other chalice #2.
- Fill beaker #1 with 300 ml of tap h2o, then add together 10 drops of i Yard NaOH. Do not spill the NaOH—information technology is very caustic!
- Make full beaker #two with 300 ml of tap water, then add iodine drops drib by drop until the solution is bright yellowish.
- Now prepare your two dialysis tubing "bags." Seal one end of each dialysis tube past carefully folding the end "hotdog fashion" ii times, and so "hamburger style" ane time. Necktie the folded portion of the tube securely with string. It is critical that your tubing is tightly sealed, to prevent leaks.
- Add together 10 ml of h2o and three drops of phenolphthalein to ane of your dialysis tube numberless. Seal the other stop of the bag by carefully folding and tying as earlier.
- Thoroughly rinse the purse containing phenolphthalein, then identify it in into the beaker containing the NaOH.
- Add 10 ml of starch solution to the other dialysis tube. Again seal the bag tightly and rinse as to a higher place. Place this bag containing the starch solution into chalice #ii.
- Allow improvidence occur between the bags and the solutions in the beakers.
- After 10 minutes, detect the color changes in the two bags and the external solutions. Depict a picture of each system below.

Data
Record the colors (below) and characterization contents inside and outside the numberless (in a higher place):
| Beaker 1 | Beaker 2 | |||
|---|---|---|---|---|
| Initial | Final | Initial | Concluding | |
| Colour inside purse | ||||
| Colour outside bag (in beaker) | ||||
Lab Questions
- Which substance diffused across the membrane in beaker #one? How do you know?
- Which substance diffused across the membrane in beaker #2? How do y'all know?
- Why might some ions and molecules pass through the dialysis bag while others might not?
Part 3: Osmosis and the Cell Membrane
Osmosis is the motion of water across a semipermeable membrane (such as the cell membrane). The tonicity of a solution involves comparing the concentration of a jail cell's cytoplasm to the concentration of its environment. Ultimately, the tonicity of a solution can be determined by examining the effect a solution has on a prison cell within the solution.
By definition, a hypertonic solution is one that causes a prison cell to compress. Though it certainly is more circuitous than this, for our purposes in this form, we can assume that a hypertonic solution is more concentrated with solutes than the cytoplasm. This will cause h2o from the cytoplasm to leave the jail cell, causing the prison cell to shrink. If a cell shrinks when placed in a solution, then the solution is hypertonic to the cell.
If a solution is hypotonic to a cell, so the cell will slap-up when placed in the hypotonic solution. In this example, you lot can imagine that the solution is less concentrated than the prison cell's cytoplasm, causing h2o from the solution to menstruum into the cell. The prison cell swells!
Finally, an isotonic solution is one that causes no change in the cell. Y'all can imagine that the solution and the cell take equal concentrations, so there is no net move of water molecules into or out of the cell.

In this exercise, you will observe osmosis by exposing a plant cell to table salt water.
Prediction
What exercise you call up will happen to the cell in this environment? Draw a film of your hypothesis.
Materials
- Elodea leaf
- Microscope slide
- Coverslip
- 5% NaCl solution
Procedure
- Remove a foliage from an Elodea plant using the forceps.
- Make a wet mountain of the leafage. Use the pond h2o to make your wet mount.
- Observe the Elodea cells nether the compound microscope at high ability (400 Ten) and depict a typical prison cell below.
- Next, add several drops of five% table salt solution to the edge of the coverslip to permit the salt to diffuse under the coverslip. Observe what happens to the cells (this may crave yous to search around along the edges of the foliage). Look for cells that have been visibly contradistinct.
Results
Draw a typical cell in both pond and salt water and label the prison cell membrane and the cell wall.
Lab Questions
- What practise y'all see occurring to the cell membrane when the cell was exposed to salt h2o? Why does this happen?
- Describe the terms hypertonic, hypotonic and isotonic.
- How would your observations change if NaCl could hands laissez passer through the cell membrane and into the cell?
Part 4: Experimental Design
You and your group will pattern an experiment to decide the relative molecular weights of methylene blue and potassium permanganate. You may apply a petri dish of agar, which is a jello-similar medium made from a polysaccharide found in the cell walls of reddish algae. You volition too have access to a cork borer and a small plastic ruler.
Materials
- 1 petri dish of agar
- Methlylene blueish
- Potassium permanganate
- Other?
Blueprint
Your experiment design should include all of the following portions:
- Hypothesis
- Experimental design
- Data
- Conclusions
- Further questions/other comments
Source: https://courses.lumenlearning.com/biolabs1/chapter/diffusion-and-osmosis/
0 Response to "Ap Biology Lab 01 Diffusion and Osmosis Video Review Sheet Answers"
Post a Comment